|
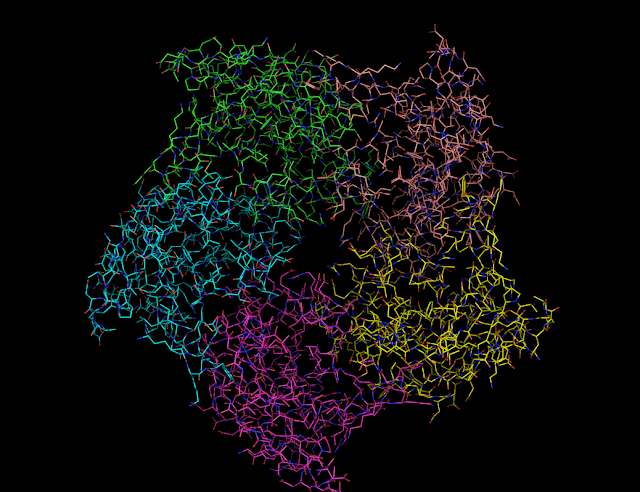
| Riboflavin Synthase 1I8D |
Riboflavin Synthase exist in a homotrimer form. It's structure consist of two beta barrel domains with a C-terminus alpha helix. Each monomer then contains two active sites. However, only one riboflavin molecule can be formed at a time for each monomer as the other is forced to face outwards towards the solvent. (1)
 | ||||
| Riboflavin Synthase monomer 1KZL |
 |
| Riboflavin Synthase monomer with Substrate 1KZL |
One of the surprising facts about this enzyme is that it is not normally found in humans. As we are able to take in riboflavin from the environment, where as bacteria are unable to. This has lead scientist to believe then that riboflavin synthase could be a potential antibacterial drug. By inhibiting this enzyme, gram negative bacteria would be unable to produce riboflavin and subsequently die. With this in mind, researchers have developed a number of inhibitors with the most effective being 9-D-ribityl-1,3,7-trihydropurine-2,6,8-purinetrione (shown below). (2)
This inhibitor works via competitive inhibition with the substrate for riboflavin synthase 6,7-dimethyl-8-ribityllumazine. It is also important to note here that riboflavin synthase often acts as a bifunctional enzyme with lumazine synthase. Thus, when these inhibitors were being made the researchers were looking at the effects it has on the overall complex. (2)
 | |
| Riboflavin Synthase/Lumazine Synthase Complex 1RVV |
 |
| Complex in an icosahedral symmetry 1VSW |
The reaction this enzyme catalyzes is:
(2) 6,7-dimethyl-8-ribityllumazine → riboflavin + 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione.The mechanism for this has been solved by researchers. In this mechanism, a nucleophile is added to one molecule of 6,7-dimethyl-8-ribityllumazine, which then goes under nucleophilic attack. Two elimination reactions then occur and finally the intermediate aromatizes through another elimination reaction to yield the products. (3)




 | |
| N-Terminal Bonding of Riboflavin Synthase with Substrate (4) |
 |
| C-Terminal Bonding of Riboflavin Synthase and Substrate (4) |
As you can see this enzyme has a extremely complex mechanism. However, it is necessary to the life of gram negative bacteria. I hope you now can see the beauty of this protein, and its huge significance towards developing antibacterial drugs.
(1) Liao DI, Wawrzak Z, Calabrese JC, Viitanen PV, Jordan DB (May 2001). "Crystal structure of riboflavin synthase". Structure 9 (5): 399–408
(2) Cushman M, Yang D, Kis K, Bacher A (December 2001). "Design, synthesis, and evaluation of 9-D-ribityl-1,3,7-trihydro-2,6,8-purinetrione, a potent inhibitor of riboflavin synthase and lumazine synthase". J. Org. Chem. 66 (25): 8320–7.
(3) Fischer M, Schott AK, Kemter K, Feicht R, Richter G, Illarionov B, Eisenreich W, Gerhardt S, Cushman M, Steinbacher S, Huber R, Bacher A (December 2003). "Riboflavin synthase of Schizosaccharomyces pombe. Protein dynamics revealed by 19F NMR protein perturbation experiments". BMC Biochem. 4: 18.
(4) http://en.wikipedia.org/wiki/Riboflavin_synthase